
OS is a package of programs to calculate the occluded surface and atomic packing of protein model structures.
Occluded surface is defined as the molecular surface that is less than 2.8 Angstroms from the surface of neighboring non-bonded atoms. That is, if a water molecule cannot fit between two atoms they occlude each other. Occluded surface is similar to buried surface but is more sensitive to packing geometry than buried surface using a rolling probe.
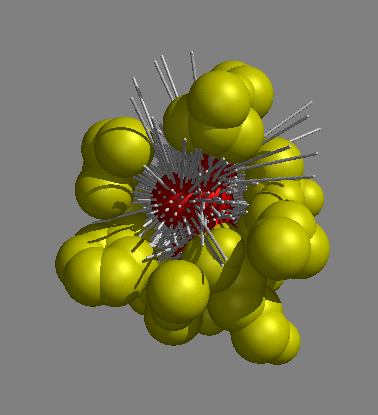
To calculate occluded surface, normals at the molecular surface are extended outward until they intersect neighboring van der Waals surface. The collection of extended normals, and their respective lengths, defines the packing of each atom in a structural model. The figure above illustrates this concept.
Analysis of the occluded surface enables one to identify amino acid residues in unusual occluded surface environments compared to a database of high resolution structures. Residues in an unusual environment are frequently incorrectly modeled, involved in crystal contacts, or involved in ligand binding.
A weighted parameter, Psr, related to the occluded surface is within a narrow range for native protein structures; if a protein model has a Psr value outside this range the model is probably wrong in at least some areas.
Analysis of the collection of extended normals (raylengths) and their associated occulded surface enables an estimation of the packing of the protein both at the residue level and for the protein as a whole. The output list identifies all atoms which interact with each atom in the structure, the surface area involved in the interaction and the average raylength for that interacting patch of surface area.
A combination of occluded surface area and average length of the normals may be used to obtain the Occluded Surface Packing (OSP) value for each residue. These residue OSP values are useful for identifying regions of close packing versus areas of loose packing in a protein.
Analysis of inter-chain occluded surface allows a detailed calculation of protein-protein interactions.
Input
Use of the program requires four files:
1. A PDB file of coordinates. This must be a 'clean' file with no alternate conformations; no hydrogens; and only one chain. You should renumber your multi-chain files to be sequentially numbered, otherwise the output will be unintelligible because chain identifiers are not kept.
A script to clean up a PDB file is described here.
A simple FORTRAN program to renumber your clean PDB file is included in the OS distribution.
How to handle RNA, DNA, prosthetic groups and other ligands is described here.
2. A command file, os.run
A sample file is in the OS distribution. You should uncomment the appropriate lines in os.run to run the different programs in the package.
An example of os.run is here.
3. A data file, os.fil, which tells OS the PDB filename, the residue numbers to calculate, and whether or not you want a file to display the extended normals using MidasPlus.
An example of os.fil with further explanation is here.
4. An atom radii file, radii
A default radii file is supplied and will be read by the program. If you want to use your own radii set, edit the file, radii, in the data/ subdirectory of the OS distribution.Default atom radii precede residue specific atom radii in the list.
If the above have been done, to start simply type:
./os.run
2. Evaluation of occluded surface environment.
3. Evaluation of the packing of the model.
4. Evaluation of interchain occluded surface.
5. Display of the extended normals using MidasPlus.
If results derived from the calculation of occluded surface are published
please cite the following:
![]() Pattabiraman, N., Ward, K.B. and Fleming, P.J. (1995) Occluded Molecular
Surface: Analysis of Protein Packing, Journal of Molecular Recognition, 8:334-344.
(This is the original description of the OS method).
Pattabiraman, N., Ward, K.B. and Fleming, P.J. (1995) Occluded Molecular
Surface: Analysis of Protein Packing, Journal of Molecular Recognition, 8:334-344.
(This is the original description of the OS method).
![]() Fleming,P.J.and F.M.Richards (2000) Protein Packing:Dependence on Protein Size, Secondary Structure
and Amino Acid Composition. J.Mol.Biol. 299, 487-498. (This is the most complete description of occluded surface packing and includes packing results for a dataset of 152 proteins).
Fleming,P.J.and F.M.Richards (2000) Protein Packing:Dependence on Protein Size, Secondary Structure
and Amino Acid Composition. J.Mol.Biol. 299, 487-498. (This is the most complete description of occluded surface packing and includes packing results for a dataset of 152 proteins).
Vorobjev, Y.N. and Hermans, J. (1997) SIMS: Computation of a Smooth Invariant Molecular Surface. Biophysical Journal, 73:722-732. (SIMS is used to calculate the dot surface for OS).
An example of increased packing in the stabilization of a hyperthermophilic protein:
![]() Brian S. DeDecker, Ronan O'Brien, Patrick J. Fleming, James H. Geiger, Stephen
P. Jackson, Paul B. Sigler (1996) The Crystal Structure of a Hyperthermophilic
Archaeal TATA-box Binding Protein, J. Mol. Biol., 264, 1072 - 1084.
Brian S. DeDecker, Ronan O'Brien, Patrick J. Fleming, James H. Geiger, Stephen
P. Jackson, Paul B. Sigler (1996) The Crystal Structure of a Hyperthermophilic
Archaeal TATA-box Binding Protein, J. Mol. Biol., 264, 1072 - 1084.
A correlation of OS with the energetics of protein-protein interaction is described in the
following paper:
![]() Fleming, KG, Ackerman, AL and DM Engelman (1997) "The Effect of Point Mutations
on the Free Energy of Transmembrane alpha-Helix Dimerization" J. Mol. Biol. 272: 266-275.
Fleming, KG, Ackerman, AL and DM Engelman (1997) "The Effect of Point Mutations
on the Free Energy of Transmembrane alpha-Helix Dimerization" J. Mol. Biol. 272: 266-275.
OS was used to show that most NMR structures have different packing than X-ray crystal structures in the following report:
![]() Ratnaparkhi et al. (1998) Discrepancies between the NMR and X-ray Structures of Uncomplexed Barstar: Analysis Suggests That Packing Densities of Protein Structures Determined by NMR Are Unreliable, Biochemistry, 37, 6958-6966.
Ratnaparkhi et al. (1998) Discrepancies between the NMR and X-ray Structures of Uncomplexed Barstar: Analysis Suggests That Packing Densities of Protein Structures Determined by NMR Are Unreliable, Biochemistry, 37, 6958-6966.
A correlation of OS with the energetics of cavity formation in proteins is described in the
following paper:
![]() Ratnapharkhi, G.S. and Varadarajan, R. (2000) "Thermodynamic and Structural Studies of Cavity Formation in Proteins Suggest That Loss of Packing Interactions Rather Than the Hydrophobic Effect Dominates the Observed Energetics.
Biochemistry 39: 12365-12374.
Ratnapharkhi, G.S. and Varadarajan, R. (2000) "Thermodynamic and Structural Studies of Cavity Formation in Proteins Suggest That Loss of Packing Interactions Rather Than the Hydrophobic Effect Dominates the Observed Energetics.
Biochemistry 39: 12365-12374.
Bug reports to Pat Fleming are appreciated.